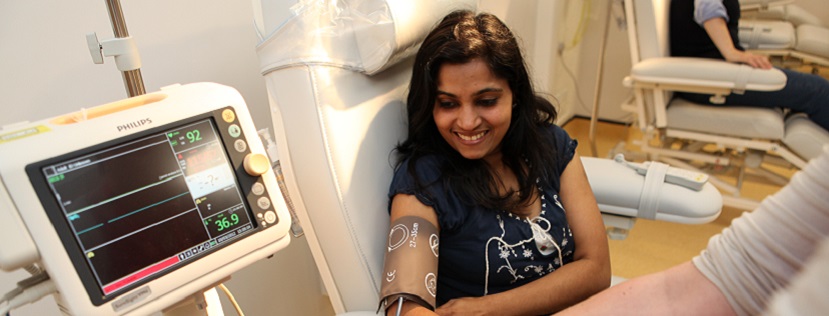
Our world-class specialist research facilities include the NIHR-Wellcome Trust King’s Clinical Research Facility (CRF), possibly the only such facility worldwide designed especially to facilitate clinical studies in mental health and brain sciences research.
The NIHR/Wellcome Trust King’s CRF offers 24/7 access to state of the art infrastructure including a high dependency Unit, GMP Cell Therapy Unit, Virtual Reality, ECG and Neuroimaging suites, behavioural assessment and biological sampling facilities. It supports Phase 0 / first-in-man to Phase 2a trials through its NIHR funded Experimental Medicine Facility, and has capacity to support later phase externally sponsored trials through its commercial clinical trials wing.
Across King’s Health Partners organisations, we offer capacity to support the full range of clinical trial phases from Phase 1 volunteer studies to Phase 3 multi-centre international trials.
Our Centre for Innovative Therapeutics (C-FIT) provides a platform for accessing innovative clinical trials expertise through the Institute of Psychiatry, Psychology & Neuroscience at King's College London.
Our Centre for Translational Informatics provides a vehicle for development, testing, evaluation and implementation of digital interventions in mental health.
Our Centre for Neuroimaging Sciences provides an interdisciplinary research environment with world-leading expertise in application-orientated brain imaging, analysis and clinical expertise for the definition, diagnosis and treatment of neurological and psychiatric disorders.
We have access to a diverse range of well-characterised research-ready cohorts and healthy controls, which means that we can deliver trials and other well designed studies quickly and efficiently:
- Our world-leading Clinical Record Informatics Search (CRIS) provides regulated, secure access to over 300,000 de-identified electronic health records, enhanced by sophisticated natural language processing capabilities.
- Through South London and Maudsley NHS Foundation Trust's innovative Consent for Contact (C4C) initiative, we have over 20,000 patients currently registered from our clinical services, who have given permission to be approached for research participation.
- The NIHR Maudsley BioResource for Mental Health and Neurology (part of the national NIHR BioResource initiative) provides access to over 10,000 deep phenotyped, re-contactable participants.
- Our BioResource is complemented by a comprehensive suite of methodological support and biomedical technology platforms, including high throughput ‘-omic’ technologies spanning genomics, proteomics and metabolomics research
- Our wide-ranging external research partnerships – including with UK Biobank, IMANOVA (now Invicro) and Genomics England's 100,000 Genome Project – allow us to capitalise on both national and local resources.
Our wide-ranging research support services offer comprehensive, streamlined support for trial management, governance and statistical services.
Our King’s Health Partners Clinical Trials Office have a Commercial Team who provide a single interface for those wishing to conduct trials sponsored by the pharmaceutical and allied healthcare industries across King’s Health Partners organisations.
The Clinical Trials Office Quality Team supports investigators at King’s Health Partners institutions who undertake clinical trials where King’s Health Partners are sponsor or co-sponsor, to ensure delivery of the statutory obligations contingent on sponsorship of trials.
Our UKCRC-registered King’s Clinical Trials Unit is available to provide support to academic led trials across King’s Health Partner organisations and externally.