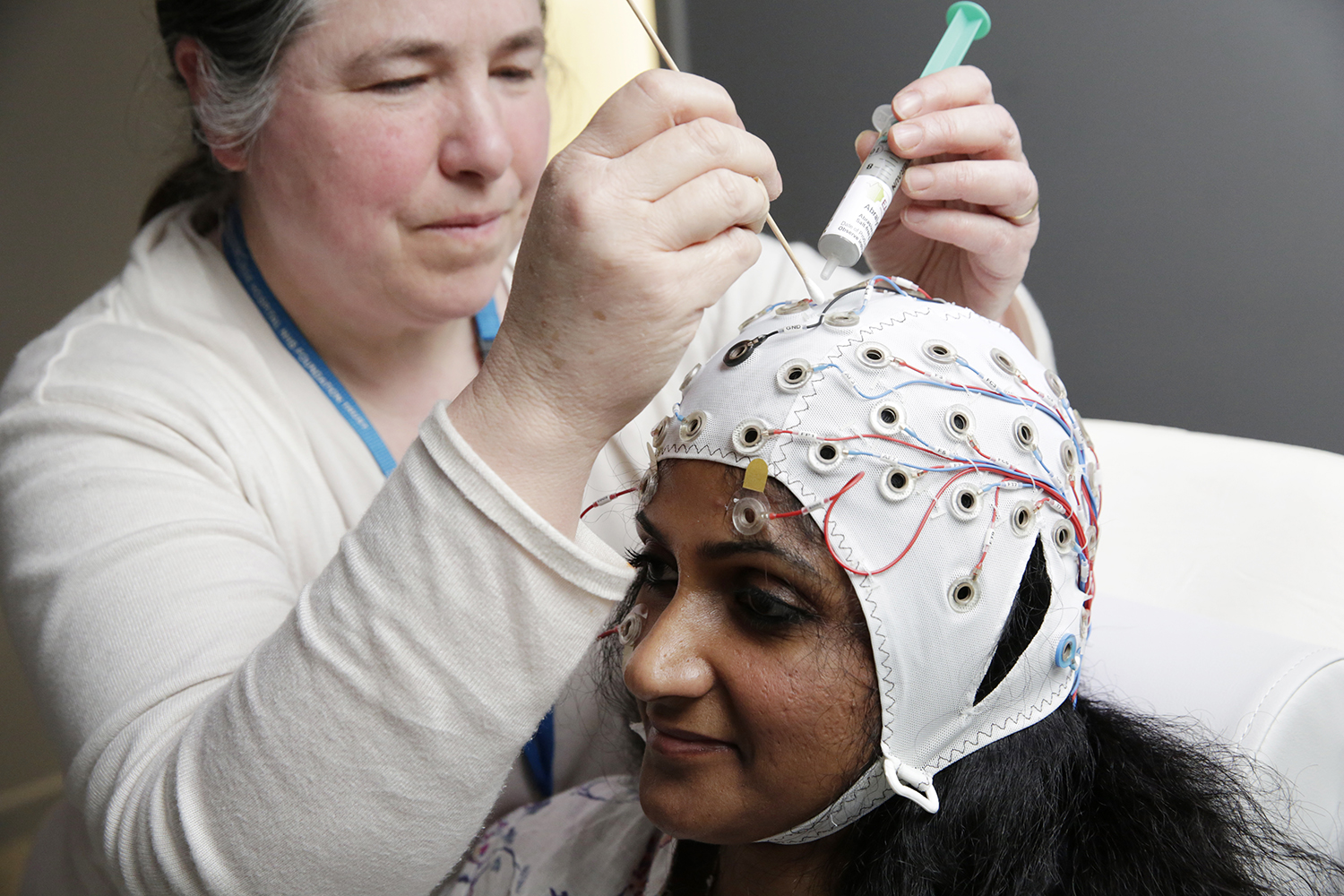
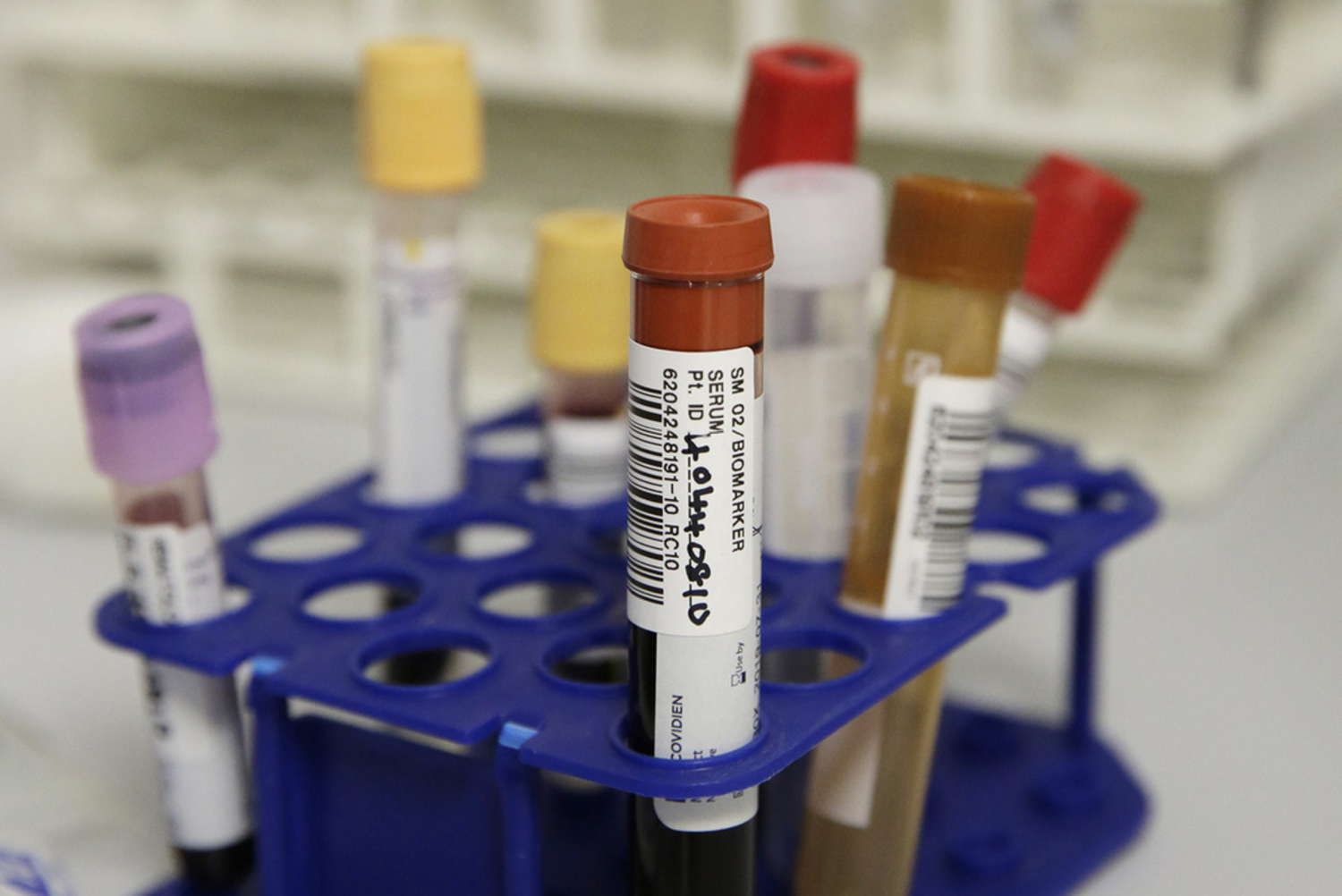
The NIHR King's Clinical Research Facility (CRF) provides dedicated facilities to support the delivery of externally-funded early translational (experimental medicine) research, including early-phase studies (Phase 2a and earlier) and early translational research studies that are nested within later-phase studies.
We are keen to facilitate the work of both new and established investigators. We also provide an environment in which the pharmaceutical industry can collaborate actively with clinical investigators.
The King's CRF works to Good Clinical Practice (GCP) and Good Manufacturing Practice (GMP) guidelines. We have processes in place to maintain the standards required by these guidelines, as well as ensuring compliance with the current legislation relating to clinical research.